I. Introduction
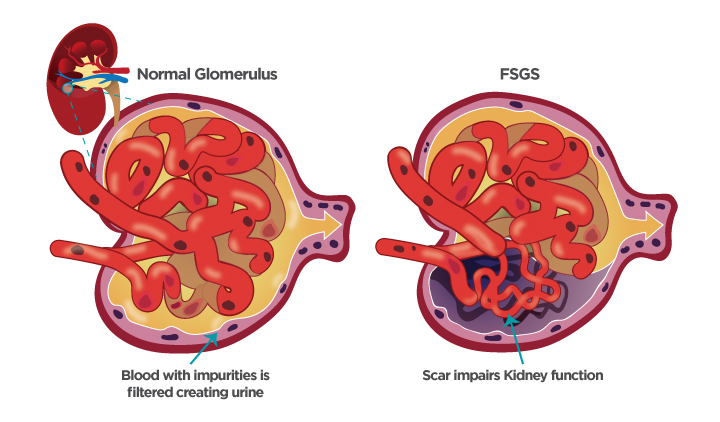
Focal segmental glomerulosclerosis (FSGS) is a rare disease characterized by scarring (sclerosis) in the filtering units (glomeruli) of the kidney. This scarring disrupts the kidney’s ability to properly filter waste and excess fluids from the blood, leading to proteinuria (excess protein in the urine), edema (swelling), and potentially, kidney failure. FSGS can be primary (idiopathic) or secondary to other conditions such as diabetes, hypertension, HIV infection, and obesity.
The global FSGS treatment market was valued at USD 23.60 billion in 2023. It is expected to grow at a compound annual growth rate (CAGR) of 8.3% during the forecast period of 2024-2032, reaching a value of USD 48.17 billion by 2032. This growth is primarily attributed to the increasing prevalence of diabetes and other kidney disorders, which are major risk factors for FSGS.
II. Market Overview
The FSGS treatment market is influenced by several factors, including the growing incidence of kidney diseases worldwide. According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), FSGS is responsible for about 2.5% of all cases of end-stage kidney disease (ESKD) in the United States. Additionally, the rising awareness about FSGS and the availability of advanced treatment options are contributing to market growth.
However, the market faces challenges such as high treatment costs and limited access to healthcare in some regions. The cost of treating FSGS can be substantial, especially for patients requiring dialysis or kidney transplantation. This can be a significant burden for patients and healthcare systems, particularly in developing countries where access to healthcare is limited.
III. Market Dynamics
One of the key trends in the FSGS treatment market is the increasing focus on developing innovative therapies. Biologics, which are drugs made from living organisms, are emerging as a promising treatment option for FSGS. These drugs target specific components of the immune system, reducing inflammation and damage to the kidneys. Gene therapies, which involve modifying genes to treat or prevent disease, are also being explored as a potential treatment for FSGS.
Regulatory agencies play a crucial role in shaping the FSGS treatment market. The approval process for new treatments can be lengthy and expensive, requiring extensive clinical trials to demonstrate safety and efficacy. Regulatory agencies also set guidelines for the pricing of treatments, which can impact their accessibility to patients.
IV. Competitive Landscape
The FSGS treatment market is highly competitive, with several companies vying for market share. Variant Pharmaceuticals, Inc., Travere Therapeutics, Inc., Sanofi S.A., and Pfizer Inc. are some of the key players in the market. These companies invest heavily in research and development to introduce new and effective treatments for FSGS.
Variant Pharmaceuticals, Inc. is a biopharmaceutical company focused on developing novel therapies for kidney diseases. Travere Therapeutics, Inc. is a biopharmaceutical company that specializes in rare diseases, including kidney disorders. Sanofi S.A. is a multinational pharmaceutical company with a strong presence in the kidney disease market. Pfizer Inc. is a leading pharmaceutical company that develops and markets a wide range of healthcare products, including treatments for kidney diseases.
Get a Free Sample Report with Table of Contents – https://www.expertmarketresearch.com/reports/focal-segmental-glomerulosclerosis-fsgs-treatment-market/requestsample
V. Market Segmentation
The FSGS treatment market can be segmented based on treatment type. Pharmacological treatments include immunosuppressants, which suppress the immune system to reduce inflammation in the kidneys. Corticosteroids, which also reduce inflammation, are another common pharmacological treatment for FSGS. Non-pharmacological options include dietary changes and lifestyle modifications, which can help manage the symptoms of FSGS and improve overall kidney health.
The market can also be segmented based on region and end-user. Hospitals and clinics are the primary end-users of FSGS treatments, as they provide the necessary medical care and support for patients with kidney diseases. Geographically, North America dominates the FSGS treatment market, followed by Europe and Asia-Pacific. The Rest of the World segment includes regions with emerging economies, where the market is expanding due to improving access to healthcare.
VI. Regional Analysis
North America is the largest market for FSGS treatments, driven by the high prevalence of kidney diseases in the region. According to the Centers for Disease Control and Prevention (CDC), more than 37 million adults in the United States have chronic kidney disease (CKD), with diabetes and hypertension being the leading causes. Europe and Asia-Pacific are also significant markets for FSGS treatments, driven by increasing healthcare expenditure and improving healthcare infrastructure.
The Rest of the World segment includes regions such as Latin America, Africa, and the Middle East, where the market for FSGS treatments is growing due to improving access to healthcare. However, challenges such as limited resources and infrastructure can hinder market growth in these regions.
VII. Future Outlook
The future of the FSGS treatment market looks promising, with continued investments in research and development expected to yield new and more effective treatments. Advancements in personalized medicine and precision therapies are also likely to drive market growth, as they allow for targeted treatments based on the individual characteristics of patients.
However, challenges such as regulatory hurdles and pricing pressures may impact market expansion. Regulatory agencies play a critical role in ensuring the safety and efficacy of new treatments, but the approval process can be lengthy and expensive. Pricing pressures can also be a challenge, as healthcare systems and insurers seek to control costs while ensuring access to innovative treatments.
Media Contact:
Company Name: Claight Corporation
Contact Person: Jhon Roy, Business Consultant
Email: sales@expertmarketresearch.com
Toll Free Number: US +1-415-325-5166 | UK +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com